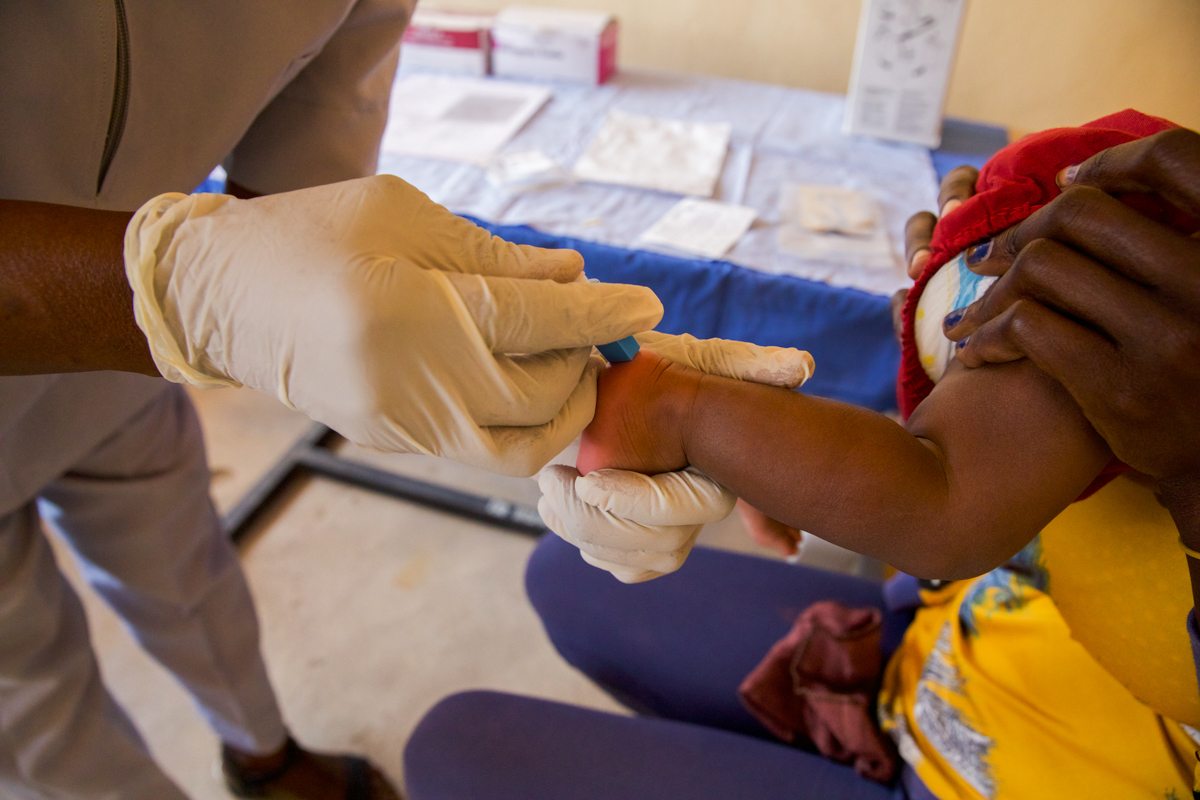
At the Conference on Retroviruses and Opportunistic Infection (CROI) in early March, Dr. Deborah Persaud announced that four children had achieved HIV remission. She sat down for an interview with EGPAF’s Eric Bond to explain the significance of this breakthrough and what it means for the fight for an AIDS-free generation.
Could you explain the significance of your announcement at CROI?
We reported that four children had gone into what we call ART-free HIV remission. ART stands for antiretroviral treatment. With HIV, once you start someone on antiretroviral treatment, that treatment is lifelong to maintain the virus from replicating.
These children were enrolled in a clinical trial of very early treatment, meaning they received antiretroviral therapy within 48 hours of life and were treated through 5-and-a-half-years of age. They then underwent what we call an analytic treatment interruption, meaning that we stopped their antiretroviral treatment, and we monitored them very closely to see if and when HIV would return in their bloodstream.
It’s called ART-free remission because these children stayed off of their antiviral drugs for more than 48 weeks without the virus bouncing back—48 weeks or more is what the clinical trial used to define ART-free remission in children.
It is crucial to understand that in almost every individual who acquires HIV—including babies and children—the virus forms a latent reservoir where it’s able to integrate its genetic material into the person’s own genetic material and stay silent. Our current therapies cannot get rid of that form of HIV, and as a result, whenever you stop therapy, the virus bounces back.
But this study demonstrates that by treating a newborn who is born with HIV within the first two days of life, remission can come about, a period of time when the virus is suppressed without the intervention of antiretroviral drugs.

How does this relate to the Mississippi baby, which was also a breakthrough for your team?
In 2013, we reported on the Mississippi baby, who was known to be infected with HIV, but who inadvertently went off treatment. Five months later, when her viral load was checked, there was no evidence of HIV in the bloodstream. There was no antibody to the virus, there was no DNA. She actually went for 27 months before the virus came back.
We identified that what was unique with the Mississippi baby was that she received a three-drug regimen at 30 hours of age.
And so we developed an NIH clinical trial—IMPAACT P1115—on the heels of this breakthrough. IMPAACT stands for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network.
In basic terms, what was the approach for the IMPAACT P1115 study?
The trial was opened in 2015 and we enrolled women who did not know their HIV status at the time that they were giving birth. We ended up enrolling 54 infants who had been confirmed to have been born with HIV. Those babies were given very early treatment regimen and monitored throughout the study until we stopped therapy—with six of them who stayed suppressed on their regimen and showed no HIV in their blood and tested negative for HIV antibody.
Now, why do we have to stop therapy? Unfortunately, there’s no test that we can do when a child or an adult is on treatment to say that that person is in remission. You have to stop therapy and continue to test to see if the virus comes back.
Four of the six children reached the 48-week benchmark. One went for 80 weeks without treatment, but then needed to be returned to treatment. We still have three who are off treatment and are still being followed.
What this means is that 11 years ago we reported on a single case of ART-free remission in a child, the Mississippi baby. And in 2024 we’ve provided the proof of concept that very early treatment of babies born with HIV from infection in utero can achieve remission.

People do not necessarily think of remission when it comes to HIV, so could you explain this and also explain how remission is different from cure?
We’re careful about using the word cure because we know that HIV persists in memory cells. Your memory cells are designed to live as long as you live, to provide you with immunologic memory, and at any point that cell can become reactivated and rekindle infection, unannounced.
For us, remission means not having to take antiretroviral drugs for some time to keep HIV in check. And the question becomes this: do we need to get to a total cure, necessarily? We are researching to see if we can get to the point where having HIV is similar to having viral infections such as the chicken pox virus that comes back as zoster? If you had chicken pox as a young child, you can then develop zoster or shingles later in life. The virus is still there, but you’re able to control it. You might have an outbreak at different times in your life, but it is controllable.
A cure, on the other hand, would be a situation where you have completely cleared every cell that has an integrated, infectious HIV genome. That’s a tall order to ask of simple treatments with conventional drugs.
In the cases of stem cell transplant, where your immune system is essentially eradicated through chemotherapy and radiation therapy and then replaced with HIV-resistant cells, I think you could venture to say that someone is potentially cured. We have seen success with this in conjunction with cancer treatment. But this is not feasible for most people living with HIV—unless you have HIV and develop cancer, of course.

Do you anticipate that early infant intervention could be scaled up to a point that this could be available for most babies who are born with HIV— potentially giving them the gift of remission?
Yes, I think so. This could be scaled up in terms of test and treat—test very early and treat very early with point-of-care tests. And those platforms are available, but they’re not accessible in most areas of the world where the burden of the infection is.
The Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) plays a large role in this—providing access to point-of-care testing where babies can be identified and placed on the very early treatment. If we can get a child treated early and virally suppressed, we hope to figure out down the road on how to transition that child to remission.
The next phase of the trial will include a different antiretroviral drug cocktail—contemporaneous regimens such as integrase inhibitors, adding immunotherapeutics. These clinical trials are costly. They are very labor intensive and require frequent monitoring.
But while the trials continue, we can start with treating kids very early. We know that very early treatment is lifesaving. What we need to figure out as a group how to get better drugs for newborns to keep them virally suppressed.
This study provided a proof of concept. We just have to do better on reaching babies earlier.
Walk us through the experience of a mother or a caregiver participating in the study.
This study was done in 11 countries around the world, most in Sub-Saharan Africa, which I think is what I’m most proud of. This is not a study that was done in the United State to benefit U.S. babies. This was done where the epidemic lies. It’s really a global effort in the pediatric community to change the lives of children and their families living with HIV.
But one thing that we often lose when we report on these findings is the investment of the mothers or guardians and their babies—because these are kids coming frequently for blood draws, in order to test their viral load.
Some of these families come from rural villages miles away to the research center to participate in this study. While the study shows the results, it doesn’t really portray the commitment of mothers and guardians and the wellbeing of their children. And somehow, we need to figure out how to capture that —because without that, we would have no outcome and no results to report on.
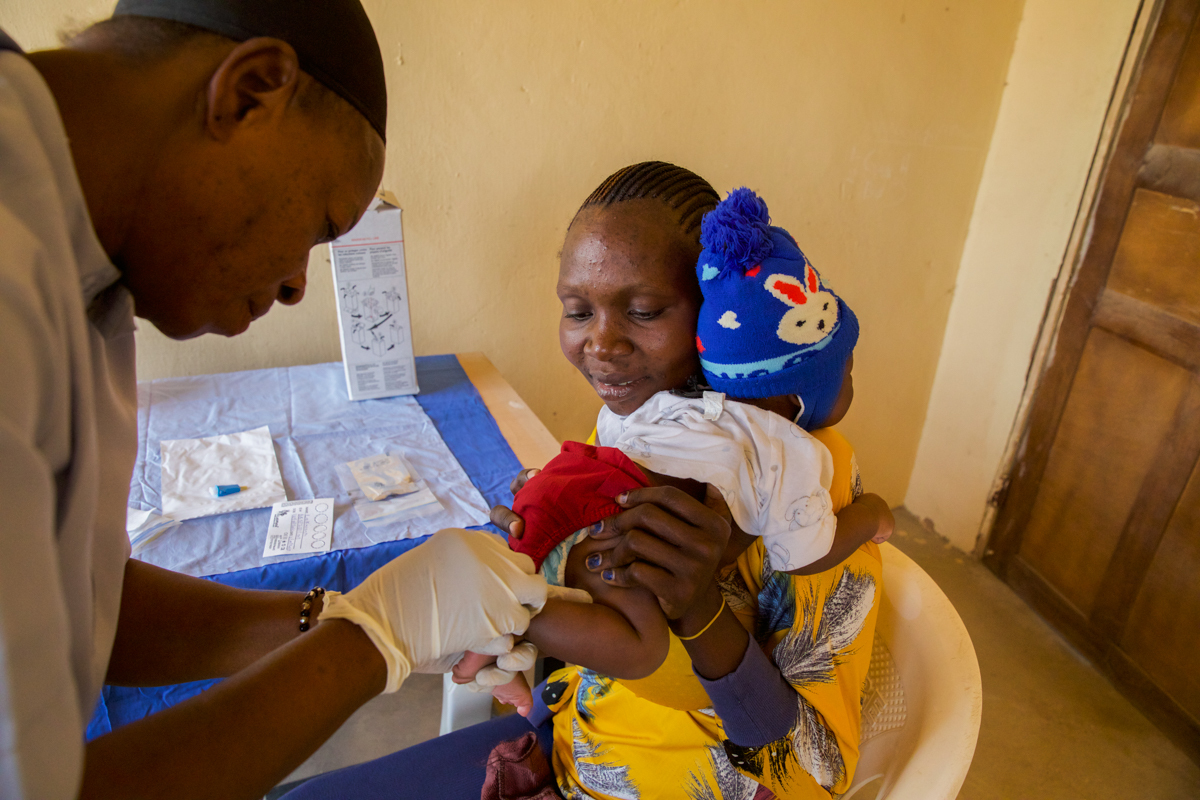
The mothers deserve a lot of credit. We have to enroll their babies right after delivery, and we have to include women who have just learned that they are living with HIV. So that’s heavy information. You’re telling a mom that she has an HIV infection and that her baby could possibly be infected.
Of course, not every child born to a mom with HIV acquires the virus. We know that the risk of a mother with untreated HIV transmitting it to her baby in utero is somewhere between 6% to 8%.
And then there is a long consent process to make sure that the mother understands the study and wants to participate. Care providers become the source of support as the moms go through the process of learning their diagnosis and being assured that there’s treatment and that with treatment a child with HIV can live a long life. If your baby does turn out to be infected, we can continue those treatments.
It is a process of giving hope around a new diagnosis. We know how to treat HIV.

Can you explain why early infant diagnosis is so important and what’s needed to make it more accessible?
We know that early treatment means a healthy immune system and is lifesaving for children. And so, it’s on us to make sure that every infant exposed to HIV is tested and treated in a timely, appropriate manner.
In most countries, because of convenience and cost, babies aren’t tested until 6 weeks of age. The turnaround time for those tests could be weeks to months. In many cases, they’re not being started on treatment until three to four months of age. We can’t operate like that anymore.
For the study, we had a system of testing babies at the time of birth. There are now near point-of-care test, with only a 90-minute turnaround time; this can revolutionize test and treat for infants.
We’ve shown that we can achieve remission in children—but the most important part was that we also showed that we can do very early testing and treatment across the globe at these 30 research sites in 11 countries.

What does this breakthrough mean to you on a personal level?
This has been an incredible journey. I first encountered pediatric HIV and AIDS during my fellowship training in New York City when many babies died, many mothers died, many fathers died. It has been a journey of decades from seeing a disease from which most children died to one where we’re talking about remission and going off therapy. It has been gratifying.
I am fortunate to be part of a large team committed to this charge. The NIH, invested in this sort of research in terms of the proof of concept. And the Elizabeth Glaser Pediatric AIDS Foundation has been pivotal for me because it provided my first funding to establish a research program, along with the Doris Duke Foundation. The Glaser Scientist program and the culture that was established through their annual meetings of bringing scientists together really allowed me to establish broad wide collaborations.
This has really been a community investment in a cause. I gather the team, and we all think together. Everyone’s doing their part with their own expertise to make this whole pie so that we can end up where we are today.
What’s next for your research team?
We have several studies underway. One is intensifying the regimens to see if we can get better outcomes—so that they block the virus’s genetic material from actually integrating into the whole cell.
Another big component we’re designing now is a clinical trial looking at a therapeutic HIV vaccine in combination with broadly neutralizing antibodies that would reduce the impact of HIV, making it more like shingles or herpes simplex in that you may get a flare up, but it subsides.
Those studies are in development, and we hope to open them by January of next year.
Deborah Persaud, M.D., is a world-renowned expert on pediatric HIV and a pediatric infectious disease specialist at the Johns Hopkins Children’s Center in Baltimore, Maryland, where she also heads the fellowship program in pediatric infectious diseases.
Dr. Persaud is the scientific chair of the HIV CURE Scientific Committee of the International Maternal, Pediatric Adolescent AIDS Clinical Trials (IMPAACT) group. She was awarded the prestigious Doris Duke Clinician Scientist and Elizabeth Glaser Scientist awards for her HIV research and was recognized by Nature magazine in 2013 as one of “Ten People Who Mattered This Year.” Also in 2013, TIME magazine named Dr. Persaud and her collaborators on the Mississippi baby case among the world’s 100 most influential people.
Dr. Persaud currently serves on EGPAF’s Board of Directors.