Lessons Learned Framework
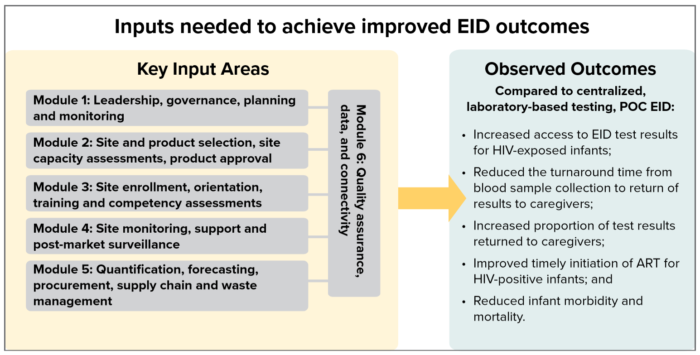
The following lessons learned modules capture key knowledge that can be used by national program managers, international implementing partners, and others to introduce or scale up point-of-care early infant diagnosis for HIV (POC EID). The modules are based on EGPAF’s experience over four years of collaborating with ministries of health and partners to introduce POC EID technologies for routine use in nine Sub-Saharan African countries. Each module suggests good practices, activities, and approaches for a specific input area (see diagram below) and includes links to guidance documents, tools, and references that can be used to support key activities described in the module.
Why POC EID?
Infants infected with HIV during pregnancy or childbirth are at a high risk of early illness and death due to their immature immune systems. Without antiretroviral therapy (ART), up to 30% of HIV-infected children will die before their first birthday, with a peak mortality risk at just 8 to 12 weeks of age. For this reason, the World Health Organization (WHO) recommends that all HIV-exposed infants have a virological test for HIV within 4 to 6 weeks of birth; that caregivers receive their child’s test results within 30 days; and that infants with an initial positive test result are immediately started on treatment. However, in 2015, fewer than 50% of HIV-exposed infants had a virological test by two months of age in the nine African countries with centralized laboratory-based testing where the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) collected baseline data. Among those tested, only 15% received their test results within 30 days. For those who received their results and were HIV-positive, only 62% started treatment within 60 days of blood sample collection.

Through funding and support from Unitaid, EGPAF leveraged its presence and experience in pediatric diagnosis, care, and treatment in nine African countries to integrate point-of-care early infant diagnosis of HIV (POC EID) into national laboratory systems. The countries were Cameroon, Côte d’Ivoire, Eswatini, Kenya, Lesotho, Mozambique, Rwanda, Zambia, and Zimbabwe. Across the nine countries, the routine use of POC testing technologies aimed to achieve national targets for pediatric HIV testing, care, and treatment by reducing gaps in EID services, with the specific objectives to:
- Increase access to EID test results for HIV-exposed infants
- Reduce turnaround time (TAT) from blood sample collection to return of test results to caregivers
- Increase the proportion of test results returned to caregivers
- Improve timely initiation of ART among HIV-positive infants
- Reduce infant morbidity and mortality
To achieve those objectives, EGPAF collaborated with national ministries of health in the nine focus countries to apply a phased implementation approach over a four-year period, which was both data-driven and sustainable. The phases included: preparation, early implementation, progressive site enrolment, routine testing and monitoring, and transitioning. Throughout all phases, EGPAF collected and routinely analyzed data and information for use not only in communications and advocacy to promote acceptance and uptake of the new technology, but also to inform the continuous quality improvement of each phase of POC EID activities. The initiative demonstrated that innovative POC technologies consistently deliver a larger proportion of test results, faster, allowing for the rapid diagnosis of more HIV-positive infants, and the initiation of ART at a younger age as compared to centralized lab-based testing.